|
|
General: HANTARO NAGAOKA JAPANESE PHYSICIST ALCHEMY MERCURY/GOLD BORN:AUGUST 15 1865
Scegli un’altra bacheca |
|
Rispondi |
Messaggio 1 di 19 di questo argomento |
|
Yes, in 1927, the Japanese physicist Hantaro Nagaoka succeeded in making gold by bombarding mercury with neutrons. Unfortunately the gold he made was dangerously radioactive.
It is possible to make stable gold, but only at great expense.
Gold is element 79 in the periodic table. This means that the nucleus of the gold atom contains 79 protons. Various isotopes are known, but only the one containing 118 neutrons is stable. This is known as Gold 197 (79 + 118 = 197). All the others with varying numbers of neutrons are radioactive.
Mercury has 80 protons in its nucleus. It has seven stable isotopes containing 116, 118, 119, 120, 121, 122 and 124 neutrons. The one containing 116 neutrons is known as Mercury 196 (80 + 116 = 196).
If Mercury 196 is bombarded with neutrons - by placing it next to a nuclear reactor for example, sometimes the nucleus absorbs an extra neutron - making Mercury 197, containing 80 protons and 117 neutrons.
Mercury 197 is unstable. From time to time, and entirely spontaneously, one of its protons will undergo epsilon decay - absorbing an electron and producing a neutron and a neutrino. The neutrino flies off, leaving the nucleus with 79 protons and 118 neutrons - Gold 197. Mercury 197 has a half life of 64 hours - meaning every 64 hours half of the Mercury 197 has converted to Gold 197, and after another 64 hours half of the remaining Mercury has converted.
The problems are that Mercury 196 is quite rare - only 0.15% of naturally occuring Mercury is Mercury 196. Irradiating it with neutrons produces other products besides Mercury 197 and separating the Mercury 197, or the Gold 197 is difficult.
All in all it is not an economic way to make gold.
In a similar way, it is also possible to make gold by irradiating element 78 - platinum - but for similar reasons that route is uneconomic also.
https://www.quora.com/Did-someone-ever-figure-out-how-to-make-gold-in-modern-times
|
|
|
|
Rispondi |
Messaggio 5 di 19 di questo argomento |
|
El Milagro de Hiroshima: Jesuitas sobrevivieron a la bomba atómica gracias al Rosario
 Hiroshima después de la bomba. (Foto: Dominio público)
Ante al 76 aniversario de los ataques atómicos a las ciudades japonesas de Hiroshima y Nagasaki, la Iglesia recuerda un episodio documentado por historiadores y médicos que es conocido como el Milagro de Hiroshima.
El 6 de agosto de 1945, fiesta de la Transfiguración, cuatro sacerdotes jesuitas alemanes sobrevivieron al impacto de la bomba nuclear “Little Boy” en Hiroshima durante la Segunda Guerra Mundial.
Los jesuitas Hugo Lassalle, superior en Japón, Hubert Schiffer, Wilhelm Kleinsorge y Hubert Cieslik, se encontraban en la casa parroquial de la iglesia de Nuestra Señora de la Asunción, uno de los pocos edificios que resistió a la bomba. En el momento de la explosión, uno de ellos se encontraba celebrando la Eucaristía, otro desayunaba y el resto en las dependencias de la parroquia.
Según escribió el propio P. Hubert Cieslik en su diario, únicamente sufrieron daños menores producto de cristales rotos, pero ninguno a consecuencia de la energía atómica liberada por la bomba.
Los médicos que los atendieron tiempo después les advirtieron que la radiación recibida les produciría lesiones graves, así como enfermedades e incluso una muerte prematura.
El pronóstico nunca se cumplió. No desarrollaron ningún trastorno y en 1976, 31 años después del lanzamiento de la bomba, el P. Schiffer acudió al Congreso Eucarístico de Filadelfia (Estados Unidos) y relató su historia, donde confirmó que los cuatro jesuitas estaban aún vivos y sin ninguna dolencia.
Fueron examinados por decenas de doctores unas 200 veces a lo largo de los años posteriores y no se halló en sus cuerpos rastro alguno de la radiación.
Los cuatro religiosos nunca dudaron de que habían gozado de la protección divina y de intercesión de la Virgen: “Vivíamos el mensaje de Fátima y rezábamos juntos el Rosario todos los días”, explicaron.
Además, el P. Schiffer escribió el libro “El Rosario de Hiroshima” donde narra todo lo que vivió.
Hace unos años, al celebrarse un aniversario más de la bomba de Hiroshima, el Obispo de Niigata, Mons. Tarcisius Isao Kikuchi, difundió un mensaje en el que subrayó que Japón puede contribuir a la paz “no con nuevas armas, sino con sus actividades de nobleza y amplia historia en el crecimiento mundial, de modo particular en las consideradas naciones en vía de desarrollo”.
El Prelado añadió que “con esta contribución al desarrollo, que lleva al pleno respeto y a la realización de la dignidad humana, sería muy apreciado y respetado por la comunidad internacional”. Cada año, del 5 al 15 de agosto, el país celebra una Oración por la Paz.
En Hiroshima y Nagasaki murieron unas 246 mil personas, la mitad en el momento del impacto de las bombas y el resto en las semanas posteriores por los efectos de la radiación.
La bomba de Hiroshima fue arrojada el dia de la Solemnidad de la Transfiguración del Señor y la rendición de Japón ocurrió el 15 de agosto, cuando la Iglesia celebra la Solemnidad de la Asunción de la Virgen María.
|
|
|
|
Rispondi |
Messaggio 6 di 19 di questo argomento |
|
Jesuit Fr. Arrupe, who ministered to victims in Hiroshima. on road to canonization

Though originally from Spain, he finished school and was ordained in Kansas, and later found himself in a Japanese prison.Imagine being in a strange land, arrested by security forces and thrown into a cold, dark cell. You are in solitary and do not know what your fate will be. You fear the executioner will be coming for you.
A few frightening days pass, and you hear footsteps approaching. Outside your cell door, muffled voices can be heard. You pray, awaiting death. But it is Christmas Day in the year 1941 in Japan. Instead of gunshots, you hear Christmas carols. You start to cry. The experience fills you with an inner peace and all-consuming love of God that remains with you for the rest of your life.
This was the experience of Pedro Arrupe, a Jesuit ministering in a land at war with the land he’d most recently called home.
Arrupe was born in the Basque region of Spain in 1907. After graduating from high school, he moved to Madrid to attend medical school. He attended Universidad Complutense, where he met the future Nobel Prize winner for medicine, Severo Ocha. After a short time, he joined the Jesuits.
When the Spanish Republican government expelled the Jesuits from Spain, Pedro could no longer pursue his studies for the priesthood. He moved to the Netherlands, then to Belgium, and finally to St. Louis Divinity School in St. Mary’s, Kansas. He was ordained there in 1936. Father Arrupe remained at St. Mary’s and completed his doctorate in Medical Ethics. After receiving his doctorate, he was sent to Japan as a missionary.
Shortly after the attack on Pearl Harbor, he was incarcerated. He was released from the Japanese prison 33 days after his arrest. He moved to Nagatsuka, which was on the outskirts of Hiroshima. On August 6, 1945, he heard the sirens blaring as the B-29 bomber got closer and closer.
He was waiting for the all-clear signal when all the windows and doors were blown into his house. Knocked to the floor, Father Pedro was stunned. When he finally stood and looked outside, he saw the city of Hiroshima in flames. He also saw the first of the 200,000 casualties of the bomb.
The priest and his friends had basic food and medical supplies but no anesthetics or modern drugs. Nevertheless, of the 150 people they cared for in their makeshift hospital, only one boy died from the effects of his injuries.
In 1958 Father Arrupe was named first Jesuit provincial for Japan. Soon after, he made a visit to Latin America. He was stunned at the people who were living in such poverty while at the same time having such an intense love and devotion to Jesus.
One particular incident had a profound effect on him. After Mass, a man invited Father Arrupe to his home. It was nothing more than a hovel but the man simply wanted to share the only gift he had. He wanted the priest to watch the sunset with him. Holding hands, they watched the sun disappear from sight. Father reflected, “I have met very few hearts that are so kind.”

Read more:
Lithuanian strawberries and goat milk and two lessons in generosity
Father Pedro Arrupe was named Superior General of the Society of Jesus in 1965. It was his mission to guide the community through the changes that followed Vatican II. He had a deep concern and love for the poor, and he wanted to make sure the Jesuits focused on their needs.
His work and influence resulted in a decree from the 32nd General Congregation, called “Our Mission Today: The Service of Faith and the Promotion of Justice.” This was passed in 1975. It guided the Jesuits in practical ways to work with the poor.
This actually resulted in threats against their lives. These threats led to the murder of six priests in El Salvador in 1989. Even that did not deter the order from its commitment to the needy and downtrodden.
Father Arrupe suffered a severe stroke in 1981. He tried to continue on but by 1983 he had to retire. His final message to his community was as follows:
More than ever I find myself in the hands of God. This is what I have wanted all my life from my youth. But now there is a difference; the initiative is entirely with God. It is indeed a profound spiritual experience to know and feel myself so totally in God’s hands.
Father Pedro Arrupe died on February 5, 1991. He was 83 years old. This year, his cause for canonization was officially started and he has been declared a Servant of God.
Servant of God Pedro Arrupe, please pray for us.
https://www.pt.aleteia.org/2018/11/17/jesuit-fr-arrupe-who-ministered-to-victims-in-hiroshima-on-road-to-canonization |
|
|
|
Rispondi |
Messaggio 7 di 19 di questo argomento |
|
|
|
|
Rispondi |
Messaggio 8 di 19 di questo argomento |
|
|
|
|
Rispondi |
Messaggio 9 di 19 di questo argomento |
|
|
|
|
Rispondi |
Messaggio 10 di 19 di questo argomento |
|
|
|
|
Rispondi |
Messaggio 11 di 19 di questo argomento |
|
|
|
|
Rispondi |
Messaggio 12 di 19 di questo argomento |
|
|
|
|
Rispondi |
Messaggio 13 di 19 di questo argomento |
|
In ‘Back to the Future’ (1985) you can see a portrait of Thomas Edison in Doc’s house when Marty travels back to 1955. This was a good prop to illustrate the time period, as no modern scientist like Doc would ever respect a degenerate conman like Edison.
https://www.reddit.com/r/shittymoviedetails/comments/getr2d/in_back_to_the_future_1985_you_can_see_a_portrait/?rdt=45765
In Back to the Future, Doc displays pictures of Newton, Franklin, Edison, and Einstein in his 1955 home while discussing time travel with Marty. All men whose work influenced Doc's invention.
https://www.reddit.com/r/MovieDetails/comments/c6n0bu/in_back_to_the_future_doc_displays_pictures_of/
|
|
|
|
Rispondi |
Messaggio 14 di 19 di questo argomento |
|
|
|
|
Rispondi |
Messaggio 15 di 19 di questo argomento |
|
Projects Mercury, Gemini and Apollo
 Astronaut Buzz Aldrin poses for a photograph beside the United States flag during the Apollo 11 Extravehicular Activity (EVA) landing on the moon, 1969. This was the world's first landing on the moon. NASAProject Mercury(1961 to 1963)
The goal of Project Mercury was to determine whether humans could survive in space. Single astronauts were launched into space in the Mercury spacecraft on six missions and spent up to 34 hours in space.
Soon after, astronaut Alan B. Shepard became the first American in space when he completed a 15-minute suborbital flight. President Kennedy committed NASA to sending a man to the moon and back before the end of the '60s. Under the direction of then-Vice President (later President) Lyndon B. Johnson, Congress appropriated funds and NASA expanded its programs to achieve President Kennedy's vision [source: Garber and Launius].
Project Gemini (1965-1966)
The Gemini spacecraft carried two astronauts and could maneuver in space. Over the course of 10 missions, astronauts changed orbits, rendezvoused with other spacecraft, docked with an unmanned Agena rocket, and walked and spent long periods of time in space.
Upon completion of the Gemini program, NASA learned how to fly, live and work in space for the durations of around two weeks that were necessary to send men to the moon and back [source: Garber and Launius].
Project Apollo (1967-1972)
Apollo's primary mission was to land men on the moon, explore it and return them safely to Earth. The Apollo spacecraft carried three men and consisted of a command module (crew quarters), service module (rocket motor, fuel cells, fuel tank, maneuvering rockets, science packages and life support), and a lunar module (a two-man, two-stage independent space vehicle for landing and lifting off from the lunar surface).
The Apollo 1 mission ended with a tragic fire on the launchpad that claimed the lives of three astronauts, Virgil Grissom, Edward White and Roger Chaffee. The Apollo spacecraft was redesigned and tested in Earth orbit during Apollo 7. Apollo 8 took astronauts into lunar orbit, then Apollo missions 9 and 10 tested the lunar module in earth orbit and lunar orbit, respectively. Apollo 11 carried the first men (Neil Armstrong and Edwin "Buzz" Aldrin) to the lunar surface, while a third astronaut (Michael Collins) orbited the moon in the command module. Armstrong and Aldrin spent hours walking on the moon, and their mission fulfilled President Kennedy's challenge.
NASA sent six more missions to explore various places on the moon, where astronauts spent up to two days exploring the lunar surface and gathering samples of moon rocks. One mission, Apollo 13, did not make it to the moon because an explosion crippled the spacecraft along the route. NASA showed its ability to handle a crisis as the agency improvised solutions to get the spacecraft around the moon and return the crew safely to Earth [source: Garber and Launius].
https://science.howstuffworks.com/nasa5.htm |
|
|
|
Rispondi |
Messaggio 16 di 19 di questo argomento |
|
Earth from Space – Arc de Triomphe, Paris
Status Report
May 13, 2022

Arc de Triomphe, Paris.
ESA
This striking, high-resolution image of the Arc de Triomphe, in Paris, was captured by Planet SkySat – a fleet of satellites that have just joined ESA’s Third Party Mission Programme in April 2022.
The Arc de Triomphe, or in full Arc de Triomphe de l’Étoile, is an iconic symbol of France and one of the world’s best-known commemorative monuments. The triumphal arch was commissioned by Napoleon I in 1806 to celebrate the military achievements of the French armies. Construction of the arch began the following year, on 15 August (Napoleon’s birthday).
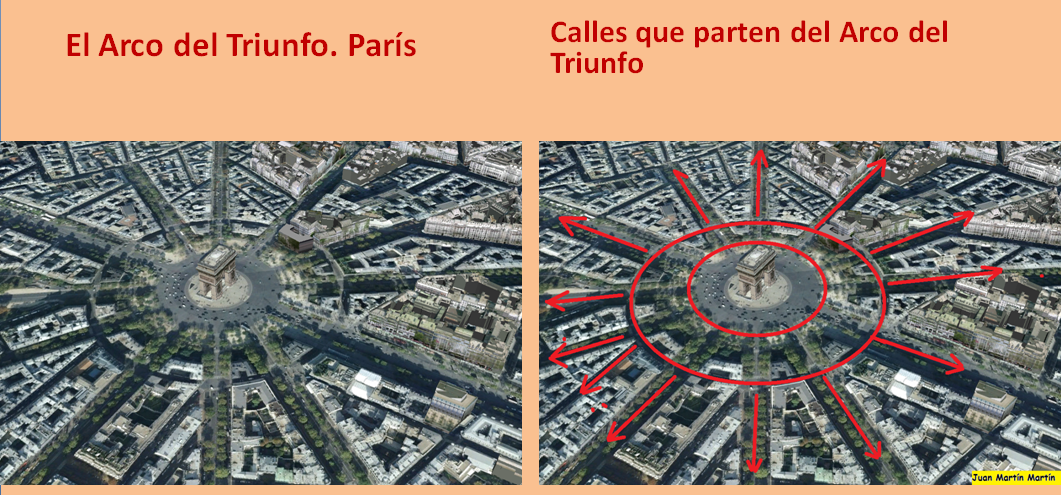
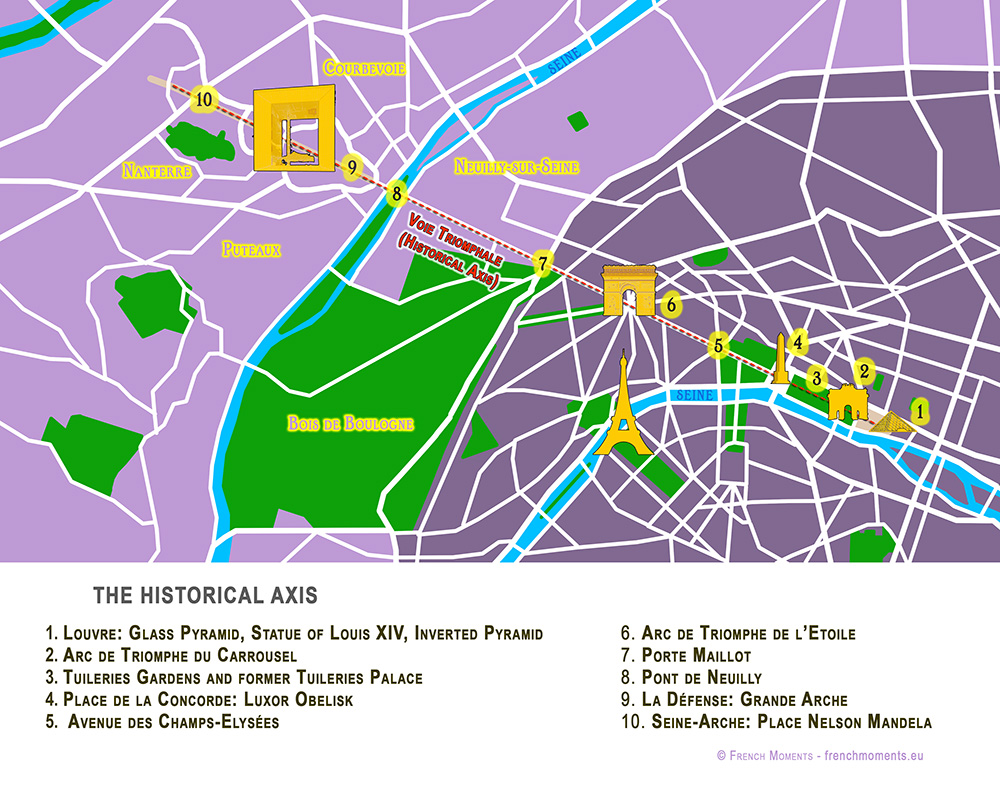
The arch stands at the centre of the Place Charles de Gaulle, the meeting point of 12 grand avenues which form a star (or étoile), which is why it is also referred to as the Arch of Triumph of the Star. The arch is 50 m high and 45 m wide.
The names of all French victories and generals are inscribed on the arch’s inner and outer surfaces, while the Tomb of the Unknown Soldier from World War I lies beneath its vault. The tomb’s flame is rekindled every evening as a symbol of the enduring nature of the commemoration and respect shown to those who have fallen in the name of France.
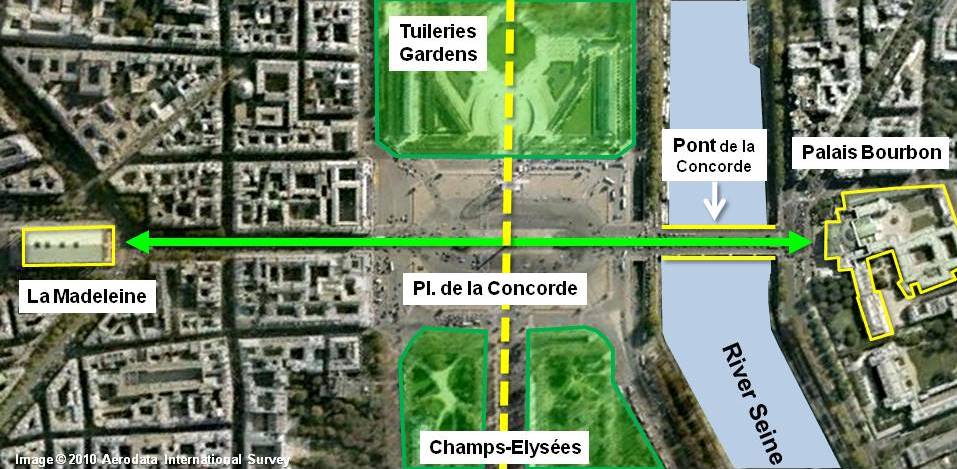
The Arc de Triomphe’s location at the Place Charles de Gaulle places it at the heart of the capital and the western terminus of the Avenue des Champs-Élysées (visible in the bottom-right of the image). Often referred to as the ‘most beautiful avenue in the world’, the Champs-Élysées is known for its theatres, cafés and luxury shops, as the finish of the Tour de France cycling race, as well as for its annual Bastille Day military parade.
This image, captured on 9 April 2022, was provided by Planet SkySat – a fleet of 21 very high-resolution satellites capable of collecting images multiple times during the day. SkySat’s satellite imagery, with 50 cm spatial resolution, is high enough to focus on areas of great interest, identifying objects such as vehicles and shipping containers.
SkySat data, along with PlanetScope (both owned and operated by Planet Labs), serve numerous commercial and governmental applications. These data are now available through ESA’s Third Party Mission programme – enabling researchers, scientists and companies from around the world the ability to access Planet’s high-frequency, high-resolution satellite data for non-commercial use.
Within this programme, Planet joins more than 50 other missions to add near-daily PlanetScope imagery, 50 cm SkySat imagery, and RapidEye archive data to this global network.
Peggy Fischer, Mission Manager for ESA’s Third Party Missions, commented, “We are very pleased to welcome PlanetScope and SkySat to ESA’s Third Party Missions portfolio and to begin the distribution of the Planet data through the ESA Earthnet Programme.
“The high-resolution and high-frequency imagery from these satellite constellations will provide an invaluable resource for the European R&D and applications community, greatly benefiting research and business opportunities across a wide range of sectors.”
To find out more on how to apply to the Earthnet Programme and get started with Planet data, click here.
– Download the full high-resolution image.
|
|
|
|
|
| Foundation stone. On August 15, 1806, Emperor Napoleon I's birthday, the foundation stone of the building was laid at a depth of eight meters, between the two southern pillars. |
|
|
|
|
| Enviado: 21/10/2024 10:30 |
|
|
|
|
|
|
|
|
|
Rispondi |
Messaggio 17 di 19 di questo argomento |
|
|
|
|
Rispondi |
Messaggio 18 di 19 di questo argomento |
|
|
|
Walter Scott
| Walter Scott |
 |
| Información personal |
| Apodo |
Border Minstrel  |
| Nacimiento |
15 de agosto de 1771
Edimburgo, Escocia, |
| Fallecimiento |
21 de septiembre de 1832 (61 años)
Abbotsford House, Melrose, Escocia |
| Causa de muerte |
Accidente cerebrovascular  |
| Sepultura |
Abadía de Dryburgh |
| Residencia |
Abbotsford House  |
| Nacionalidad |
Escocia |
| Lengua materna |
Inglés  |
| Familia |
| Padres |
Walter Scott 
Anne Rutherford  |
| Cónyuge |
Charlotte Carpenter (Charpentier) |
| Hijos |
4  |
| Educación |
| Educado en |
|
| Información profesional |
| Ocupación |
novelista, poeta, abogado, Sheriff de Selkirkshire |
| Años activo |
siglo xix |
| Cargos ocupados |
Juez  |
| Movimiento |
Romanticismo |
| Seudónimo |
Jedediah Cleishbotham, Laurence Templeton, Somnambulus, Malachi Malagrowther, Clutterbuck y Lawrence Templeton  |
| Lengua literaria |
inglés |
| Géneros |
Novela histórica, poesía, teatro y Romanticismo  |
| Obras notables |
|
| Miembro de |
|
| Distinciones |
- Baronet
- Miembro de la Sociedad Real de Edimburgo

|
| Firma |
 |
|
|
Walter Scott, primer baronet (Edimburgo, Escocia, 15 de agosto de 1771-Abbotsford House, 21 de septiembre de 1832), fue un escritor británico prolífico del Romanticismo de acción, especializado en novelas históricas, género del que se le puede considerar inventor,1 además de ser poeta y editor. Fue conocido en toda Europa en su época, y, en cierto sentido, fue el primer autor que tuvo una verdadera carrera internacional en su tiempo, con muchos lectores contemporáneos en Europa, Australia y Norteamérica.[cita requerida]
Sus novelas históricas y, en menor medida, su poesía, aún se leen, pero hoy es menos popular de lo que fue en la cumbre de su éxito. A pesar de ello, muchas de sus obras siguen siendo clásicos en la literatura inglesa y específicamente escocesa. Algunos de sus títulos más famosos son Ivanhoe, Rob Roy, The Lady of the Lake, Waverley y The Heart of Midlothian.
Aunque recordado principalmente por sus extensas obras literarias y su compromiso político, Scott fue abogado, juez y administrador legal de profesión, y a lo largo de su carrera combinó su trabajo de redacción y edición con su ocupación diaria como secretario de sesión y alguacil-diputado de Selkirkshire.
Scott, un miembro destacado del establecimiento conservador en Edimburgo, fue miembro activo de la Highland Society, sirvió durante un largo período como presidente de la Royal Society of Edinburgh (1820-1832) y fue vicepresidente de la Society of Antiquaries of Scotland (1827-1829).
Nació en College Wynd, en Edimburgo en 1771; era hijo de un abogado. El joven Walter Scott sobrevivió a un ataque de polio en su infancia que lo dejó cojo de la pierna derecha de por vida. Para restaurar su salud, lo enviaron a vivir durante varios años a la región rural de los Borders (en el sureste de Escocia, fronterizo con Inglaterra) durante siete meses para estabilizar su enfermedad. Allí vivió en la granja de sus abuelos en Sandyknowe. Aprendió el habla de la zona, así como los cuentos y leyendas que caracterizarían gran parte de su trabajo. Su estado de salud motivó también que pasara parte de su infancia en la ciudad balnearia de Bath, en Inglaterra.
Después de estudiar Derecho en la Universidad de Edimburgo, siguió los pasos de su padre y se hizo abogado en Edimburgo. Como empleado de un abogado hizo su primera visita a las Tierras Altas escocesas, para ejecutar un desahucio.
Scott estaba enamorado de Williamina Belsches de Fettercairn, a quien le había propuesto matrimonio varias veces. A pesar de que ella había sido ambigua al contestarle, Scott esperaba que tarde o temprano aceptara. Pero en 1796, Scott se fue a un viaje, y cuando regresó se dio cuenta de que Williamina se estaba enamorando de William Forbes, VII.º baronet de Pitsligo y uno de sus amigos, con quien ella terminaría casándose (más tarde tendrían al científico James David Forbes). Cuando se anunció el compromiso entre Belsches y Forbes, Scott primero se enfadó mucho con ella. Aunque Scott sufrió una decepción amorosa y un sentimiento de dolor que se quedaría en él durante un tiempo, después comprendió que ella no quería hacerle daño.23 Scott seguiría siendo amigo de Forbes, a quien después de morir, aludiría en Marmion,4 y en una carta donde lo describía como un buen amigo.5
|
|
|
|
|
|
|
Rispondi |
Messaggio 19 di 19 di questo argomento |
|
|
|
 Primo Primo
 Precedente
5 a 19 de 19
Successivo Precedente
5 a 19 de 19
Successivo
 Ultimo
Ultimo
|
|
| |
|
|
©2025 - Gabitos - Tutti i diritti riservati | |
|
|



 Hiroshima después de la bomba. (Foto: Dominio público)
Hiroshima después de la bomba. (Foto: Dominio público)


![Regreso Al Fururo III (Back To The Future III) [1990] –, 40% OFF](https://m.media-amazon.com/images/M/MV5BYzgzMDc2YjQtOWM1OS00ZjhhLWJiNjQtMzE3ZTY4MTZiY2ViXkEyXkFqcGdeQXVyNDQ0MTYzMDA@._V1_.jpg)


















































































 Astronaut Buzz Aldrin poses for a photograph beside the United States flag during the Apollo 11 Extravehicular Activity (EVA) landing on the moon, 1969. This was the world's first landing on the moon.
Astronaut Buzz Aldrin poses for a photograph beside the United States flag during the Apollo 11 Extravehicular Activity (EVA) landing on the moon, 1969. This was the world's first landing on the moon.